BioCat Perspectives/Reviews
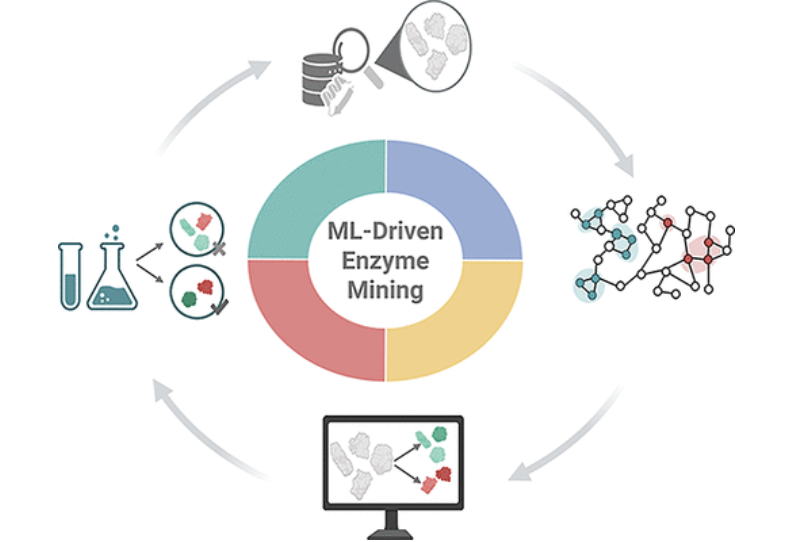
Machine Learning-Driven Enzyme Mining: Opportunities, Challenges, and Future Perspectives
Abstract: Enzyme mining is rapidly evolving as a data-driven strategy to identify biocatalysts with tailored functions from a vast landscape of uncharacterized proteins. The integration of machine learning (ML) into these workflows enables high-throughput prediction of enzyme functions─including Enzyme Commission numbers, Gene Ontology terms, and substrate specificity─as well as key catalytic properties, such as kinetic parameters, optimal temperature, pH, solubility, and thermophilicity. This review provides a systematic overview of state-of-the-art ML models and highlights representative case studies that demonstrate their effectiveness in accelerating enzyme discovery. Despite notable progress, current approaches remain limited by data scarcity, model generalizability, and interpretability. We discuss emerging strategies to overcome these challenges, including multitask learning, integration of multimodal data, and explainable AI. We outline how the convergence of machine learning, autonomous experimentation, and agentic AI systems could accelerate progress toward self-driving enzyme discovery. Together, these developments position ML-guided enzyme mining as a scalable, interpretable, and increasingly autonomous framework for uncovering biocatalysts in biotechnology, biocatalysis, and synthetic biology.
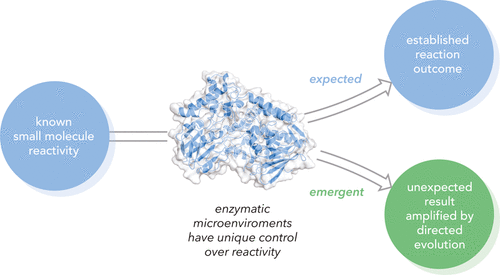
Emergent Mechanisms in Biocatalysis
Abstract: Enzymes are invaluable tools for solving challenges in synthetic organic chemistry. Beyond replicating native reactivity patterns, modern directed evolution strategies have enabled chemists to efficiently survey chemical space to identify enzyme families capable of catalyzing non-natural reactions. While methods often focus on chemo-, enantio-, and regiocontrol, there are a growing number of examples that describe reactivity patterns and reaction mechanisms that were previously unknown in the synthetic literature. In this Perspective, we will explore examples of such emergent mechanistic pathways of enzymes in the context of synthetic precedents, emphasizing the remarkable versatility of diverse enzyme active sites in controlling unprecedented transformations.
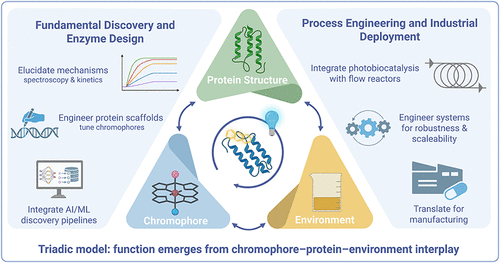
Beyond Flavoproteins: Toward the Industrialization of Photobiocatalysis
Abstract: Photobiocatalysis merges enzymatic selectivity with the synthetic versatility of light, enabling complex transformations under mild, aqueous conditions. However, advances in this field remain primarily focused on the adaptation of native flavoproteins and known oxidoreductases, such as ene-reductases, with limited exploration of non-natural cofactors or engineered proteins. This perspective examines the current progress in photobiocatalysis, in addition to design constraints that limit the scope of photobiocatalysis, including narrow cofactor compatibility, short excited-state lifetimes, and the instability of enzyme–chromophore systems under irradiation. We highlight the overreliance on a small subset of light-responsive enzyme complexes and propose a broader, modular framework for photoenzyme development. By dissecting the interplay between chromophore identity, protein structure, and reaction environment, we outline strategies to extend the reactivity, stability, and tunability of photoenzymes beyond their native roles. Together, these strategies provide a blueprint for the systematic design, benchmarking, and application of photobiocatalytic systems for broader use and industrial applications.
Enabling Broader Adoption of Biocatalysis in Organic Chemistry
Abstract: Biocatalysis is becoming an increasingly impactful method in contemporary synthetic chemistry for target molecule synthesis. The selectivity imparted by enzymes has been leveraged to complete previously intractable chemical transformations and improve synthetic routes toward complex molecules. However, the implementation of biocatalysis in mainstream organic chemistry has been gradual to this point. This is partly due to a set of historical and technological barriers that have prevented chemists from using biocatalysis as a synthetic tool with utility that parallels alternative modes of catalysis. In this Perspective, we discuss these barriers and how they have hindered the adoption of enzyme catalysts into synthetic strategies. We also summarize tools and resources that already enable organic chemists to use biocatalysts. Furthermore, we discuss ways to further lower the barriers for the adoption of biocatalysis by the broader synthetic organic chemistry community through the dissemination of resources, demystifying biocatalytic reactions, and increasing collaboration across the field.
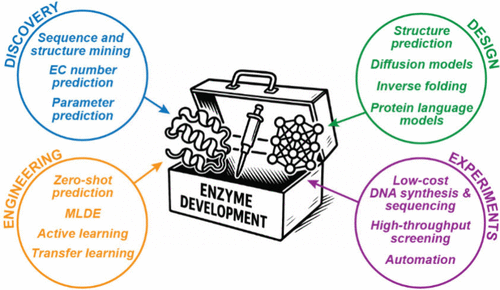
Of Revolutions and Roadblocks: The Emerging Role of Machine Learning in Biocatalysis
Abstract: Machine learning (ML) is rapidly turning into a key technology for biocatalysis. By learning patterns in amino acid sequences, protein structures, and functional data, ML models can help navigate complex fitness landscapes, uncover new enzymes in databases, and even design biocatalysts de novo. Along with advances in DNA synthesis and sequencing, laboratory automation, and high-throughput screening, ML is increasing the speed and efficiency of enzyme development. In this Outlook, we highlight recent applications of ML in the fields of enzyme discovery, design, and engineering, with a focus on current challenges and emerging solutions. Furthermore, we discuss barriers that impede a broader and faster adoption of ML-based workflows in the biocatalysis community. We conclude by suggesting best practices for fostering effective collaborations in this interdisciplinary field.
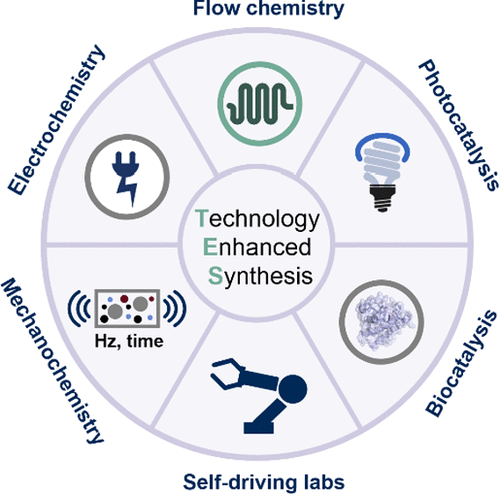
Tech-Enhanced Synthesis: Exploring the Synergy between Organic Chemistry and Technology
Abstract: Recent years have witnessed growing interest in integrating enabling technologies into synthetic organic chemistry to address long-standing challenges in reproducibility, sustainability, and scalability. This perspective showcases how modern tools, ranging from continuous-flow reactors and electrochemical cells to photochemical technologies, biocatalysis, mechanochemistry, and self-driving laboratories, are reshaping the way chemists design, perform, and optimize reactions. Through selected case studies, we highlight how these technologies not only solve specific reactivity and process issues but also open new avenues for reactivity discovery and chemical innovation. Rather than viewing technology as a complication, we advocate for its adoption as a natural extension of synthetic creativity, capable of enhancing safety, reducing waste, and expanding accessible chemical space. Our aim is to inspire broader implementation and interdisciplinary training to equip the next generation of chemists with the tools to rethink how synthesis is performed in the 21st century.
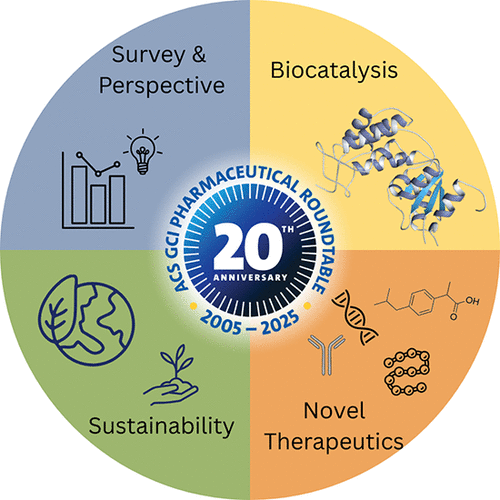
The Evolving Landscape of Industrial Biocatalysis in Perspective from the ACS Green Chemistry Institute Pharmaceutical Roundtable
Abstract: As the ACS Green Chemistry Institute Pharmaceutical Roundtable (GCIPR) approached its 20th anniversary, the Biocatalysis Focus Team surveyed its member companies to better understand how biocatalysis is currently being leveraged across their pipelines. This article presents an analysis of the dataset collected from pharmaceutical and agrochemical companies, highlighting the evolving biocatalysis landscape with expanding impact of enzyme catalysis driven by protein engineering. The increasing complexity of active pharmaceutical ingredients (APIs) demands efficient and sustainable synthesis routes, prompting the pharmaceutical industry to adopt innovative methodologies. In this context, biocatalysis has emerged as a particularly attractive solution, as it enables streamlined syntheses under mild reaction conditions with intrinsically safer reaction profiles compared with conventional chemistry. Over the past two decades, numerous API manufacturing processes have integrated biocatalysis, leveraging a wide range of enzymes in early drug discovery and route scouting activities. Advances in directed evolution, computational tools, and adjacent technologies now allow for the rapid discovery and optimization, further expanding the use of biocatalysis in pharmaceutical manufacturing.
Modeling Enzyme Kinetics: Current Challenges and Future Perspectives for Biocatalysis
Abstract: Biocatalysis is becoming a data science. High-throughput experimentation generates a rapidly increasing stream of biocatalytic data, which is the raw material for mechanistic and novel data-driven modeling approaches for the predictive design of improved biocatalysts and novel bioprocesses. The holistic and molecular understanding of enzymatic reaction systems will enable us to identify and overcome kinetic bottlenecks and shift the thermodynamics of a reaction. The full characterization and modeling of reaction systems is a community effort; therefore, published methods and results should be findable, accessible, interoperable, and reusable (FAIR), which is achieved by developing standardized data exchange formats, by a complete and reproducible documentation of experimentation, by collaborative platforms for developing sustainable software and for analyzing data, and by repositories for publishing results together with raw data. The FAIRification of biocatalysis is a prerequisite to developing highly automated laboratory infrastructures that improve the reproducibility of scientific results and reduce the time and costs required to develop novel synthesis routes.
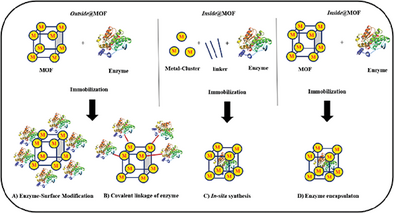
Merging MOF Chemistry & Biocatalysis: A Perspective for Achieving Efficient Organic Synthetic Processes and Applications in the Chemical Industry?
Abstract: Biocatalysis has emerged in recent decades toward a widely applied catalysis technology in the chemical industry. In particular the fine chemicals and pharmaceuticals industries benefit from the advantages of biocatalysis, which made its way to a dominating industrial core technology for manufacturing chiral molecules. However, often biocatalysis is still to a certain extent away from having solved all challenges being needed for a “perfect industrial process technology”. Among existing challenges are, e.g., stability under process conditions and easy separation of the catalyst from the reaction mixture with the additional option of recyclability. Here metal-organic framework (MOF) structures offer unique advantages, which can be beneficial for biocatalysis. A particular valuable option is integration of enzymes into MOF-subunits, thus having a potential positive impact on stability (by reducing the tendency of unfolding) and enabling compartmentalization as a beneficial strategy in, e.g., chemoenzymatic synthesis. In this “Perspective”-article, first the state of the art in biocatalysis is briefly summarized together with current challenges. Then, a short overview about current research achievements in “merging” MOF chemistry and biocatalysis is given as well as an outlook written from a biocatalysis practitioner’s view, how MOF-enzyme hybrid systems can play a major role in future process development to overcome existing hurdles of enzymatic catalysis.
Photobiocatalytic Strategies for Organic Synthesis
Abstract: Biocatalysis has revolutionized chemical synthesis, providing sustainable methods for preparing various organic molecules. In enzyme-mediated organic synthesis, most reactions involve molecules operating from their ground states. Over the past 25 years, there has been an increased interest in enzymatic processes that utilize electronically excited states accessed through photoexcitation. These photobiocatalytic processes involve a diverse array of reaction mechanisms that are complementary to one another. This comprehensive review will describe the state-of-the-art strategies in photobiocatalysis for organic synthesis until December 2022. Apart from reviewing the relevant literature, a central goal of this review is to delineate the mechanistic differences between the general strategies employed in the field. We will organize this review based on the relationship between the photochemical step and the enzymatic transformations. The review will include mechanistic studies, substrate scopes, and protein optimization strategies. By clearly defining mechanistically-distinct strategies in photobiocatalytic chemistry, we hope to illuminate future synthetic opportunities in the area.
Fundamentals, Applications, and Future Directions of Bioelectrocatalysis
Abstract: Bioelectrocatalysis is an interdisciplinary research field combining biocatalysis and electrocatalysis via the utilization of materials derived from biological systems as catalysts to catalyze the redox reactions occurring at an electrode. Bioelectrocatalysis synergistically couples the merits of both biocatalysis and electrocatalysis. The advantages of biocatalysis include high activity, high selectivity, wide substrate scope, and mild reaction conditions. The advantages of electrocatalysis include the possible utilization of renewable electricity as an electron source and high energy conversion efficiency. These properties are integrated to achieve selective biosensing, efficient energy conversion, and the production of diverse products. This review seeks to systematically and comprehensively detail the fundamentals, analyze the existing problems, summarize the development status and applications, and look toward the future development directions of bioelectrocatalysis. First, the structure, function, and modification of bioelectrocatalysts are discussed. Second, the essentials of bioelectrocatalytic systems, including electron transfer mechanisms, electrode materials, and reaction medium, are described. Third, the application of bioelectrocatalysis in the fields of biosensors, fuel cells, solar cells, catalytic mechanism studies, and bioelectrosyntheses of high-value chemicals are systematically summarized. Finally, future developments and a perspective on bioelectrocatalysis are suggested.